Al-Mg Binary Phase Diagram
Purpose: Learn to calculate and use a binary phase diagram
Module: PanPhaseDiagram
Thermodynamic Database: AlMgZn.tdb
Batch file: Example_#1.1.pbfx
Calculation Procedures:
-
Load AlMgZn.tdb following the procedure in Pandat User's Guide: Load Database , and select Al and Mg two components;
-
Perform section (2D) calculation following the procedure in Pandat User's Guide: Section Calculation (2D);
-
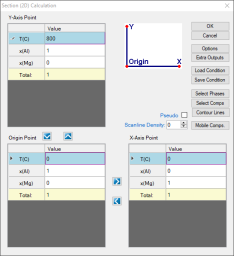
Set Calculation Condition as shown in Figure 1;
Post Calculation Operation:
-
Label phase field following the procedure in Pandat User's Guide: Icons for Graph on Toolbar;
-
Change graph appearance following the procedure in Pandat User's Guide: Property ;
-
Zoom the interested area following the procedure in Pandat User's Guide: Icons for Graph on Toolbar;
Information obtained from this calculation:
-
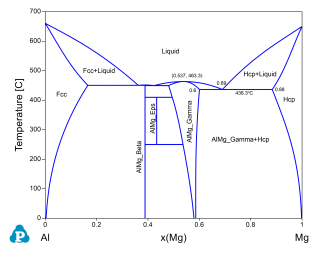
Phase stability as a function of composition and temperature;
-
Congruent melting temperature and composition of a phase. For example the congruent melting temperature of the AlMg_Gamma phase is at 463.3°C and x(Mg)=0.537;
-
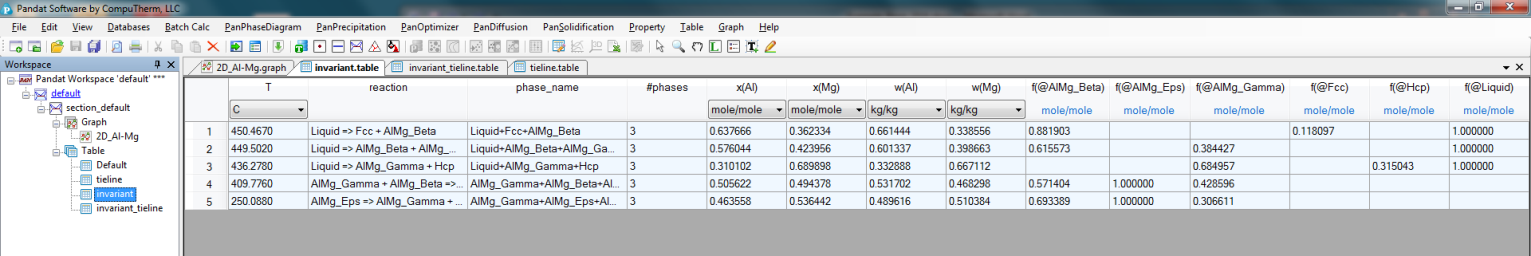
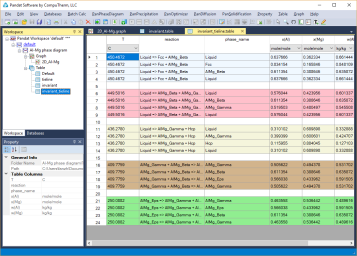
Invariant reaction temperatures and phase compositions. For example the three phase equilibrium: Liquid->AlMg_Gamma+Hcp is at 436.3°C, the composition, x(Mg), of each phase at equilibrium is indicated in Figure 2. Details on the invariant reactions can be found in the “invariant” table as shown in Figure 1, and composition of each phase involved in the invariant reaction can be found in the “invariant_tieline” tables as shown in Figure 4.